ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição
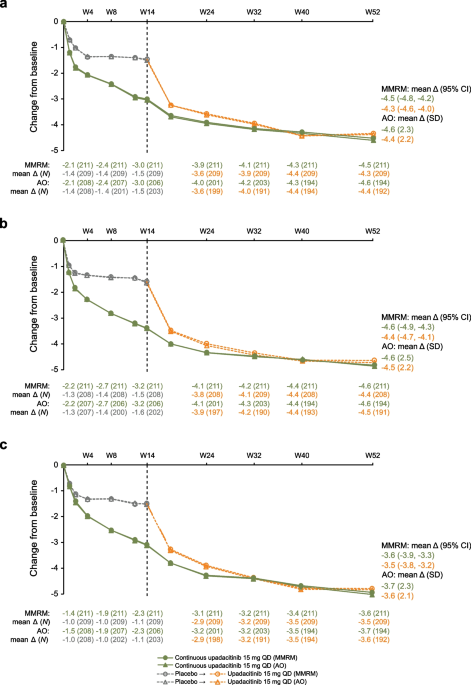
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy
Baseline predictors of (A) ASDAS Major Improvement and (B) ASAS40 at
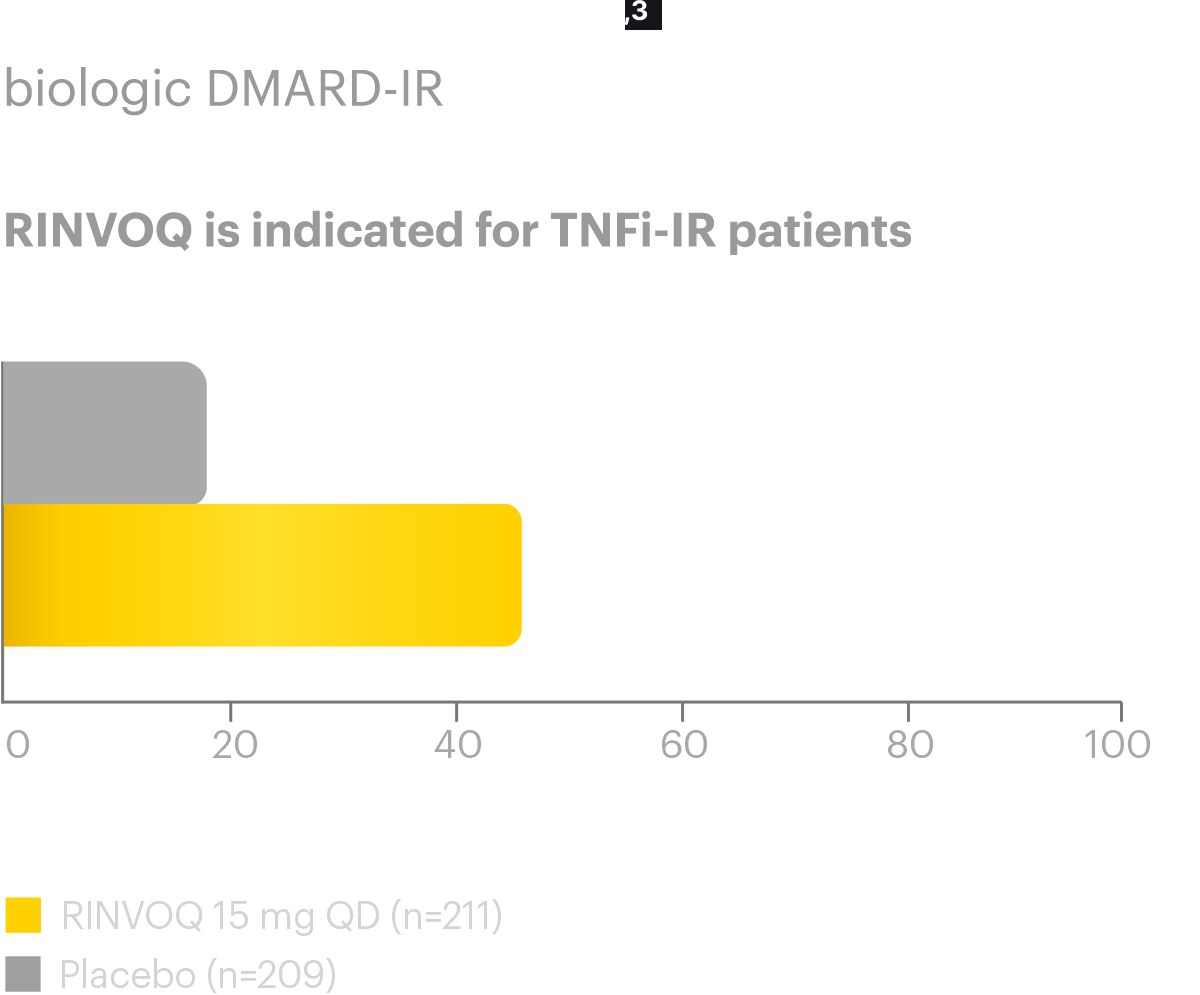
Disease Control Data, Ankylosing Spondylitis
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy

Percentages of patients achieving ASDAS LDA (

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis
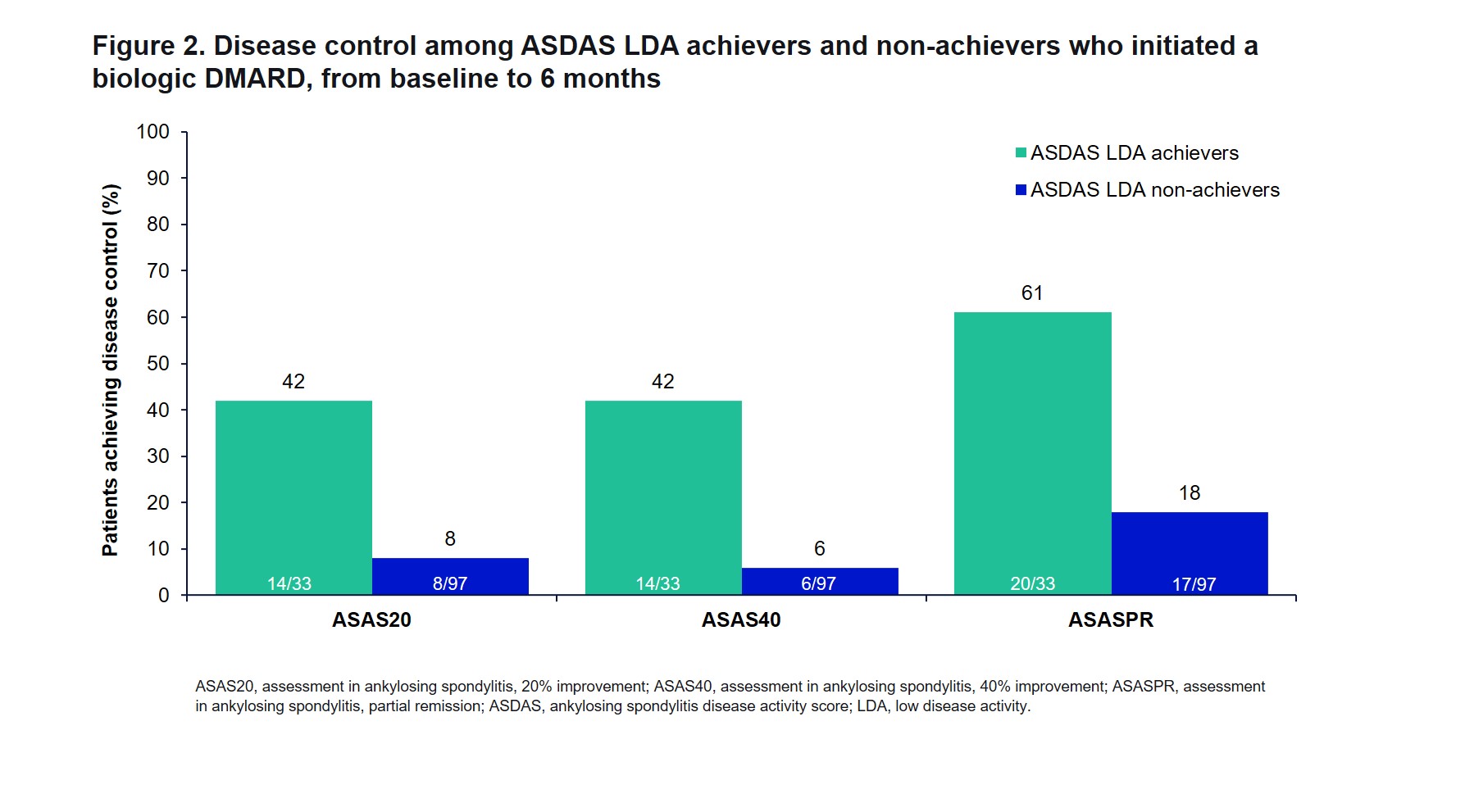
Impact of Achieving ASDAS LDA on Disease Activity and Patient-Reported Outcome Measures Among Patients with Ankylosing Spondylitis Treated with Biologic DMARDs - ACR Meeting Abstracts
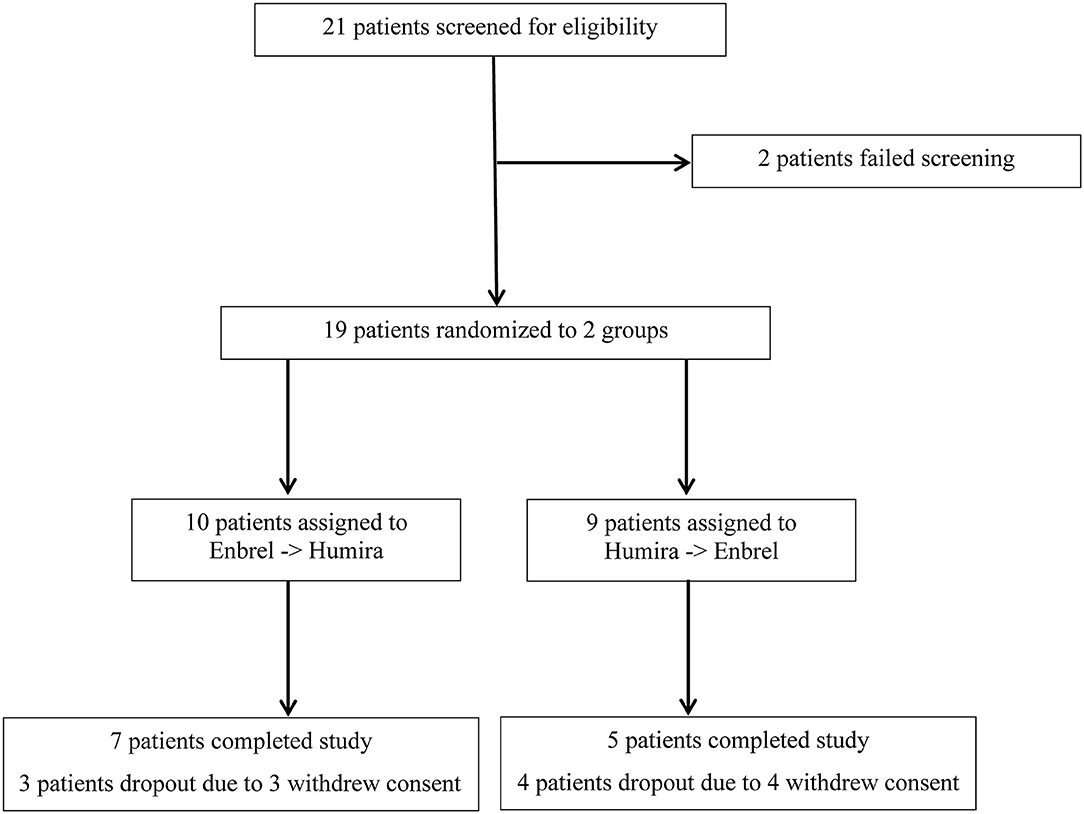
Frontiers Head-to-Head Comparison of Etanercept vs. Adalimumab in the Treatment of Ankylosing Spondylitis: An Open-Label Randomized Controlled Crossover Clinical Trial

Full article: Effectiveness of bDMARDs in ankylosing spondylitis patients by biologic use: experience from the CorEvitas PsA/SpA Registry
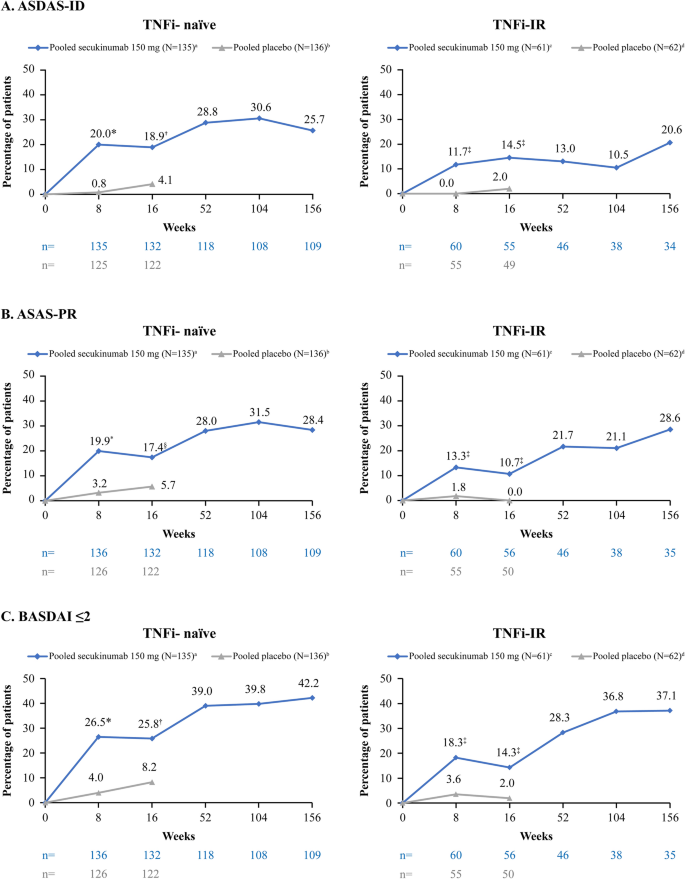
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies

AS Criteria: Diagnosis (ASAS), Disease Activity (ASDAS), Radiographic Progression (mSASSS) - Arthritis Rheumatism
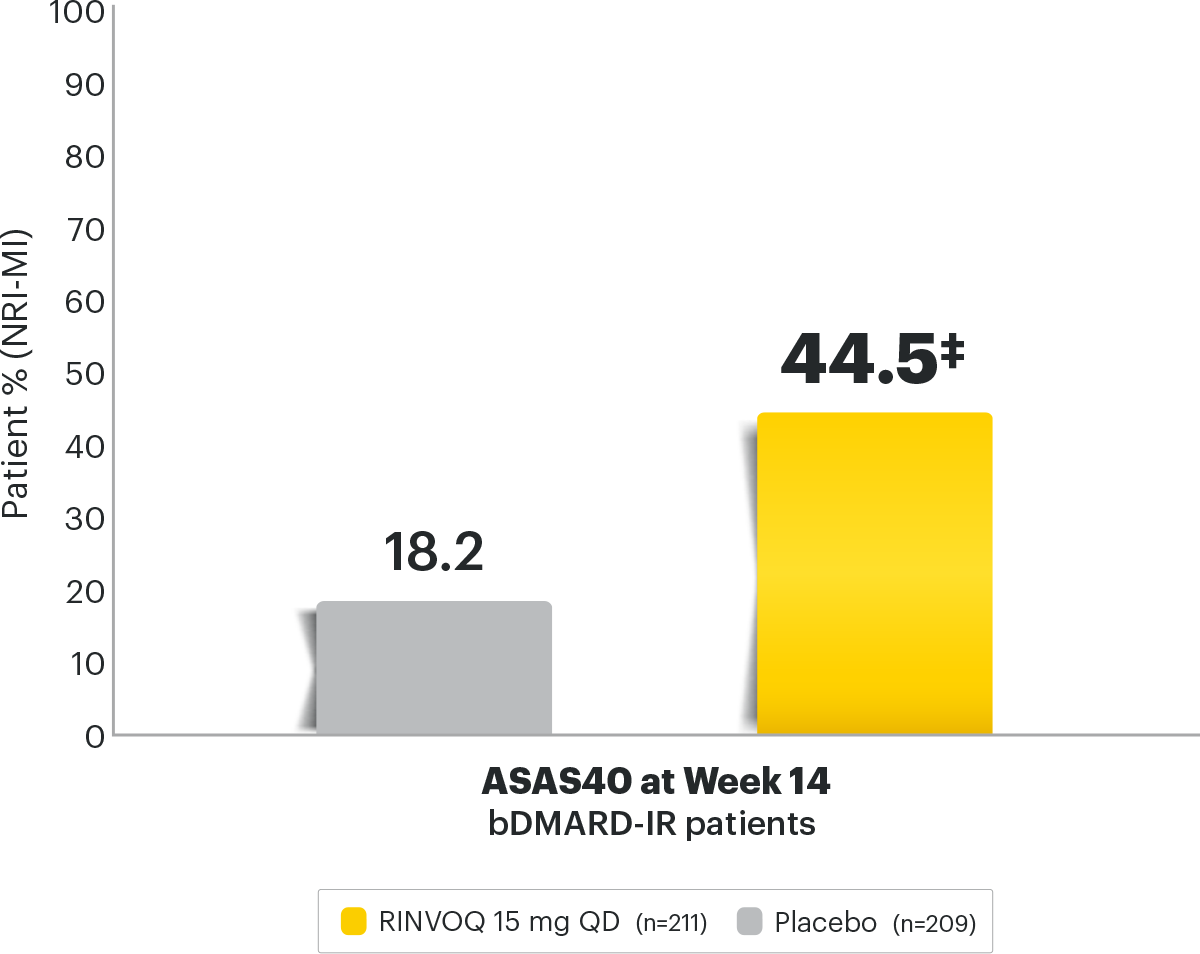
Axial Spondyloarthritis RINVOQ® (upadacitinib)

Oral Abstracts - 2020 - International Journal of Rheumatic Diseases - Wiley Online Library

Baseline characteristics of ASDAS ID and ASAS PR responders and
de
por adulto (o preço varia de acordo com o tamanho do grupo)