Performance of BASDAI vs. ASDAS in Evaluating Axial Involvement in Patients with PsA Treated with Guselkumab: Pooled Analysis of Two Phase 3 Studies - ACR Meeting Abstracts
Por um escritor misterioso
Descrição
Background/Purpose: Although the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is used to assess the activity of axial disease in patients (pts) with PsA, only one of its questions is specific to axial symptoms. Alternatively, the Ankylosing Spondylitis Disease Activity Score (ASDAS) excludes assessment of enthesitis, gives less weight to peripheral activity and is considered […]
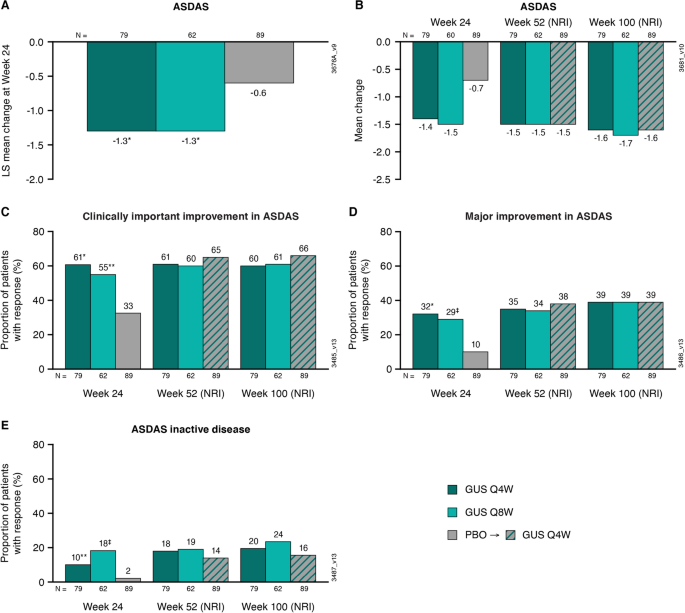
Efficacy of Guselkumab on Axial-Related Symptoms Through up to 2 Years in Adults with Active Psoriatic Arthritis in the Phase 3, Randomized, Placebo-Controlled DISCOVER-2 Study
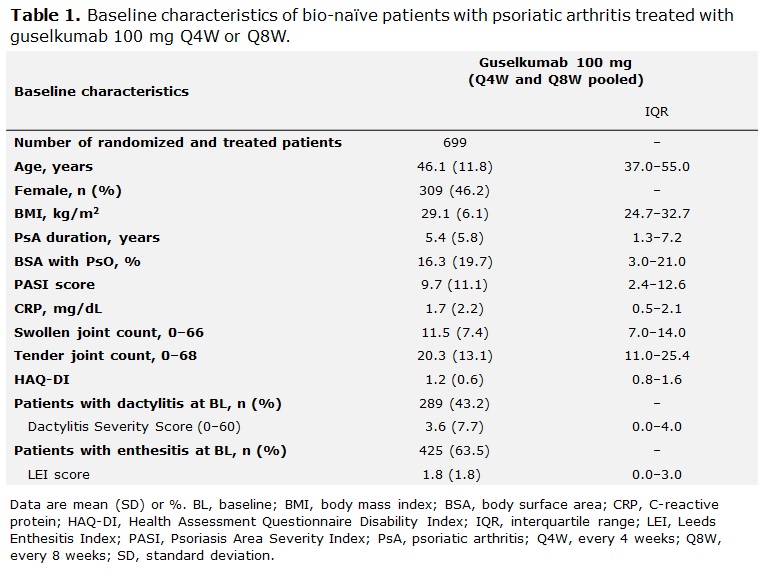
To What Extent Do Clinical Features of PsA Predict Achievement of Minimal Disease Activity at Week 24: A Post Hoc Analysis of the Phase III Clinical Trial Program of Guselkumab in a
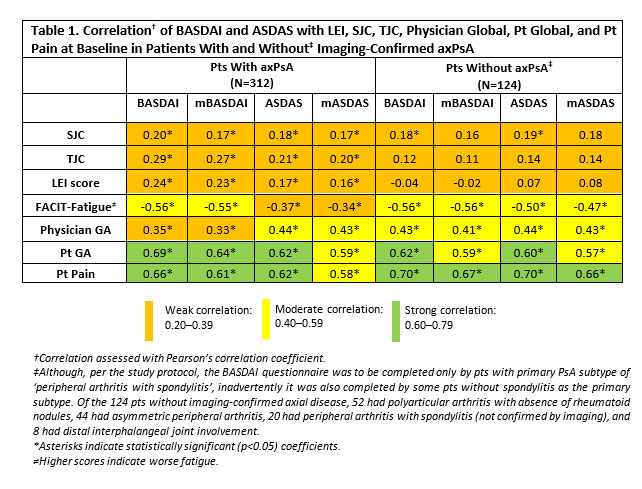
Performance of BASDAI vs. ASDAS in Evaluating Axial Involvement in Patients with PsA Treated with Guselkumab: Pooled Analysis of Two Phase 3 Studies - ACR Meeting Abstracts

PDF) The effect of ixekizumab on axial manifestations in patients with psoriatic arthritis from two phase III clinical trials: SPIRIT-P1 and SPIRIT-P2

PDF) Efficacy of Guselkumab on Axial-Related Symptoms Through up to 2 Years in Adults with Active Psoriatic Arthritis in the Phase 3, Randomized, Placebo-Controlled DISCOVER-2 Study

PDF) Axial involvement in psoriatic arthritis: An update for rheumatologists

PDF) Identification of PsA phenotypes with machine learning analytics using data from two phase III clinical trials of guselkumab in a bio-naïve population of patients with PsA
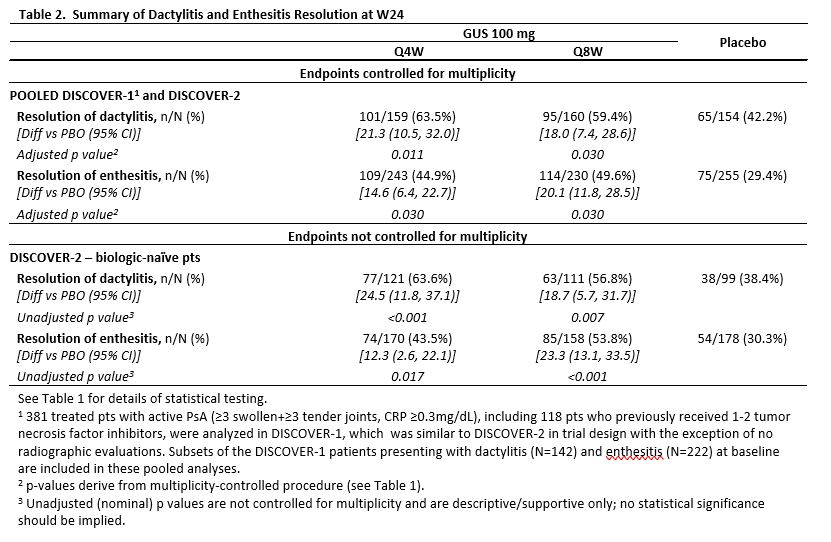
Guselkumab, an Anti-interleukin-23p19 Monoclonal Antibody, in Biologic-naïve Patients with Active Psoriatic Arthritis: Week 24 Results of the Phase 3, Randomized, Double-blind, Placebo-controlled Study - ACR Meeting Abstracts

Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial - The Lancet
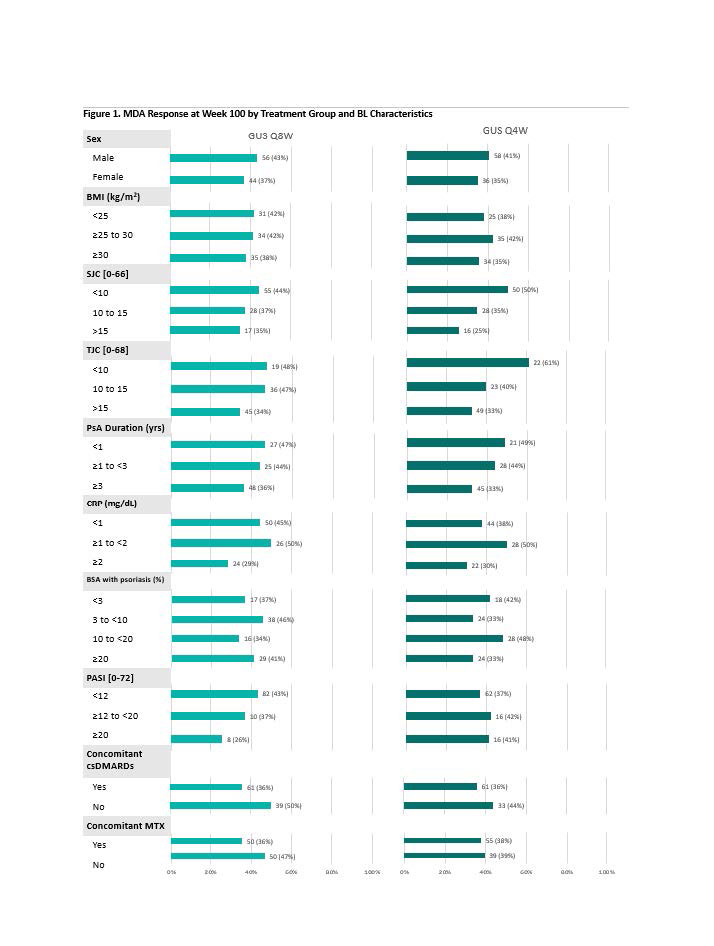
Stringent Disease Activity Control at 2 Years Across Psoriatic Arthritis Domains Irrespective of Baseline Characteristics in Patients Treated with Guselkumab: Post Hoc Analysis of a Phase 3, Randomized, Double-blind, Placebo-controlled Study
de
por adulto (o preço varia de acordo com o tamanho do grupo)