What Does the IRB Review?, Research
Por um escritor misterioso
Descrição
Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.
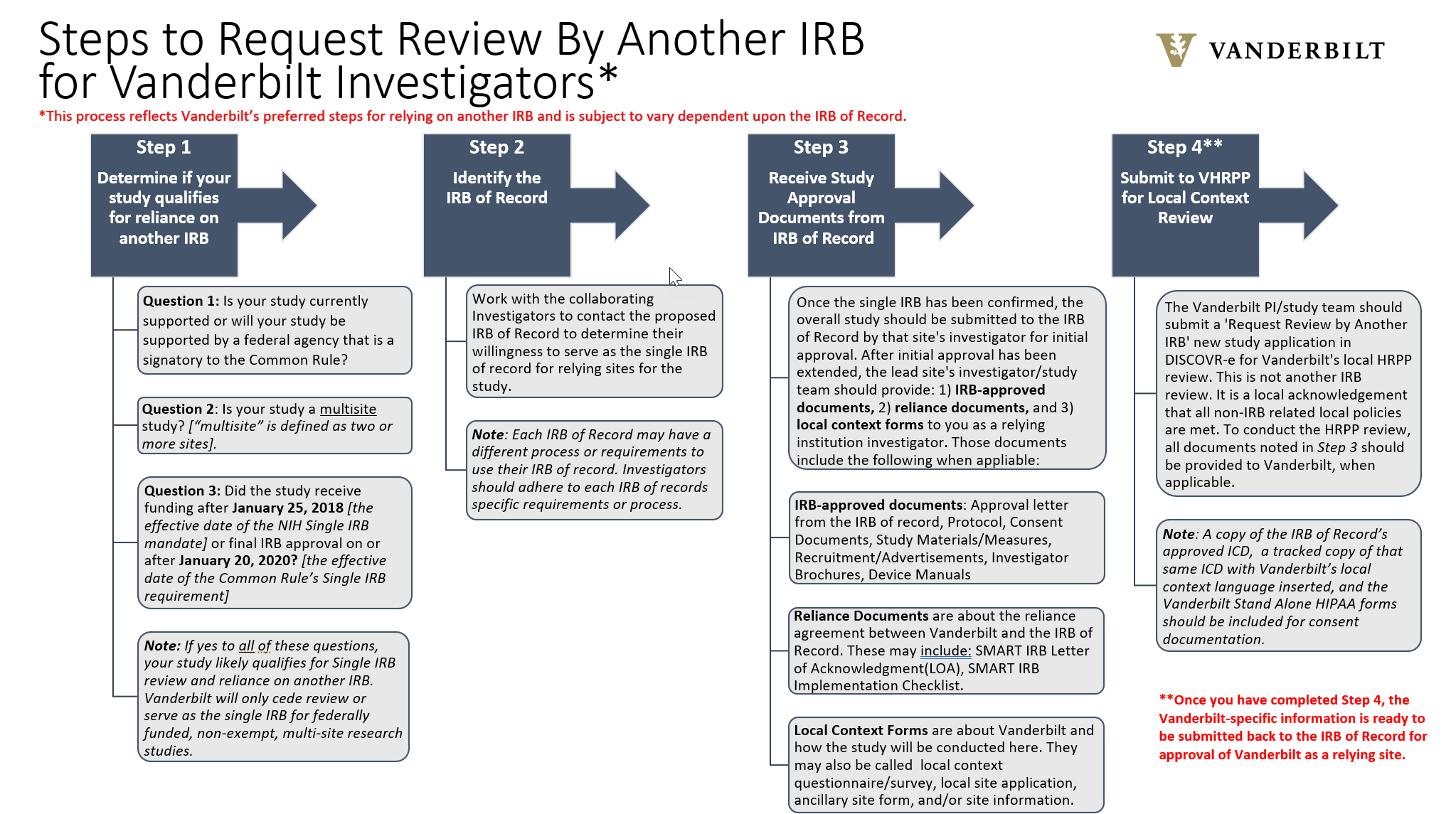
Single IRB Help Human Research Protections Program
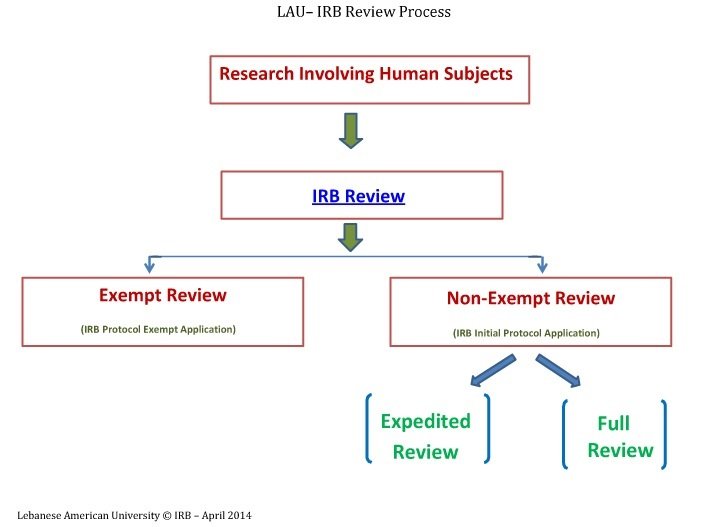
IRB Types of Review, Ethical Compliance, Graduate Studies and Research
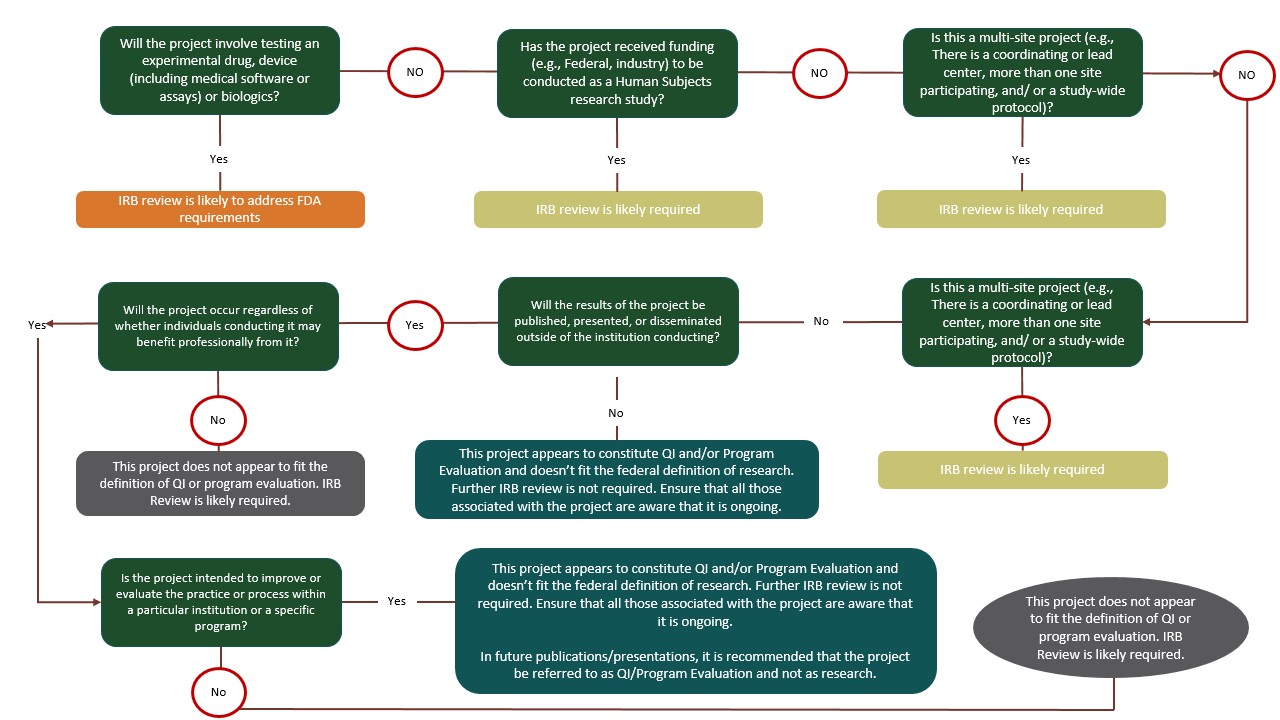
FAQs - Vice President For Research

PDF) The reporting Of IRB review in journal articles presenting HIV research conducted in the developing world
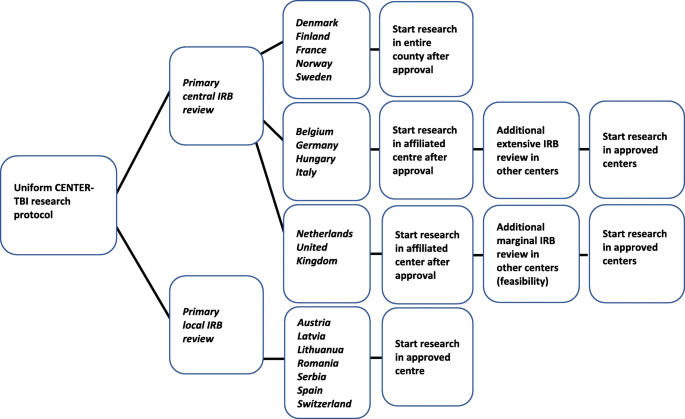
How do 66 European institutional review boards approve one protocol for an international prospective observational study on traumatic brain injury? Experiences from the CENTER-TBI study, BMC Medical Ethics
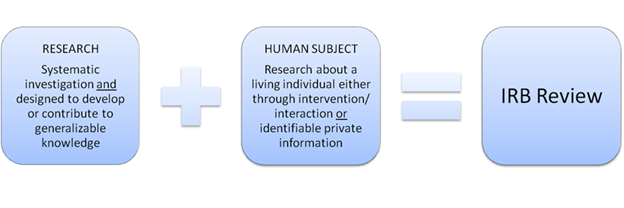
Human Subjects Research
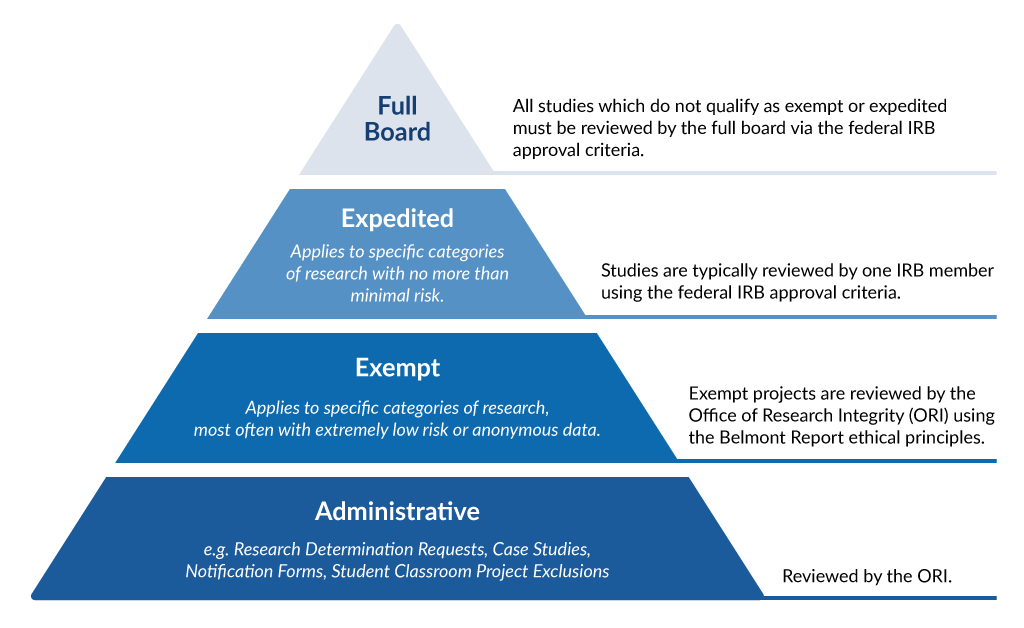
Frequently Asked Questions University of New England in Maine

Webinar: What You Should Know About IRB Review of Research

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions
/research.png?n=4093)
Institutional Review Board Governors State University
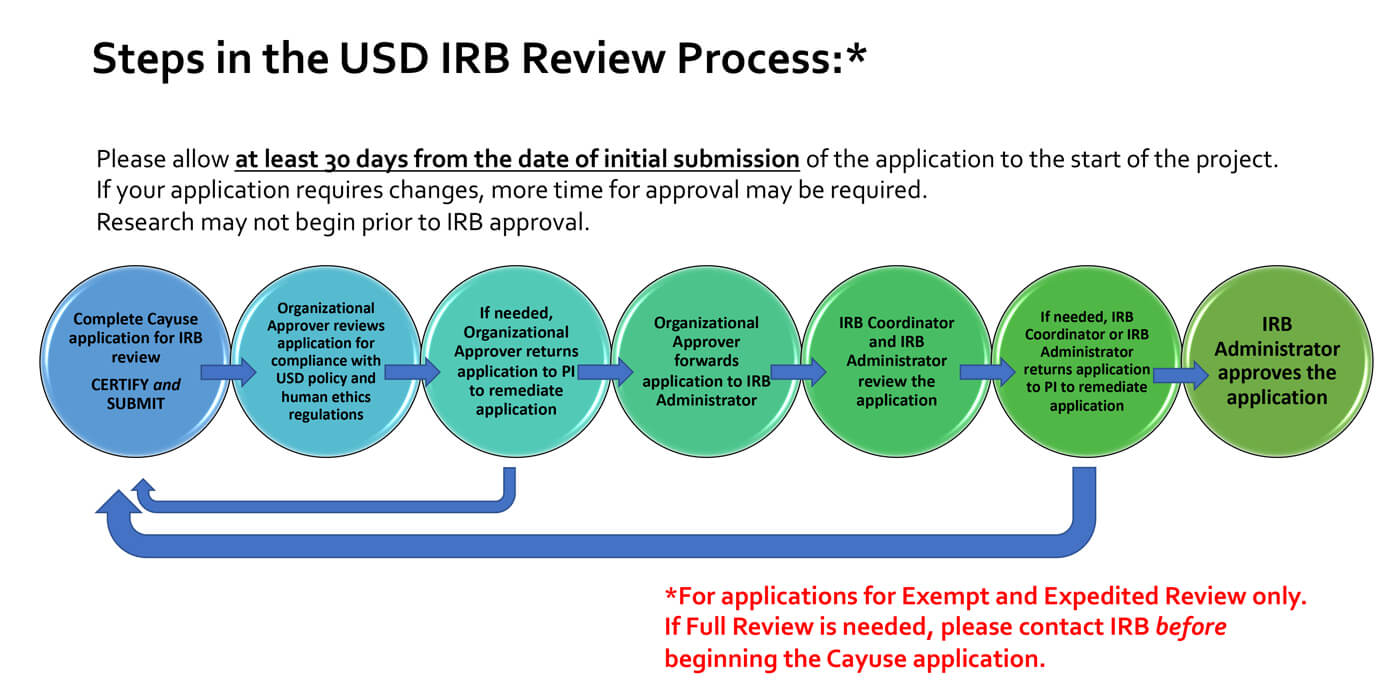
Getting Started: Prior to Applying - Institutional Review Board - University of San Diego
de
por adulto (o preço varia de acordo com o tamanho do grupo)







