What You Should Know About CSV in Pharma
Por um escritor misterioso
Descrição
Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.
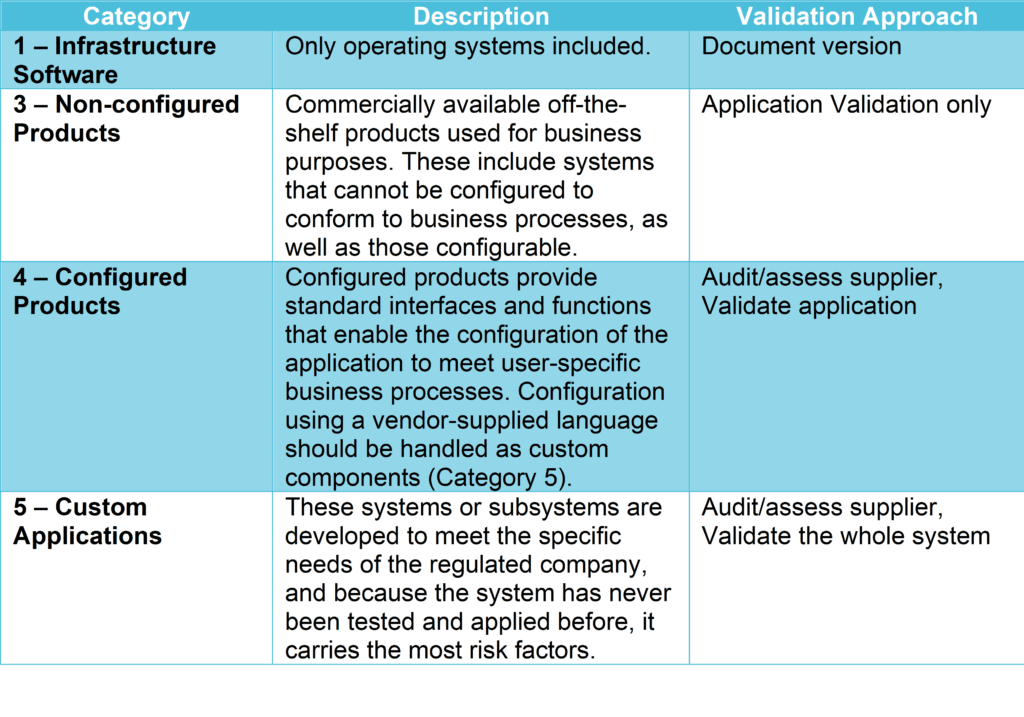
Risk-Based Computerized System Validation (CSV) and Computer Software Assurance (CSA) - Old Wine in a New Bottle? - Kvalito

Computer System Validation (CSV): Your Path to Documenting Data Integrity

Computerized System Validation for Pharmaceutical Organizations: A foundation guidebook eBook : Sharma, Ashish K: : Books

Software Validation: Here's How We Do It - Apprentice
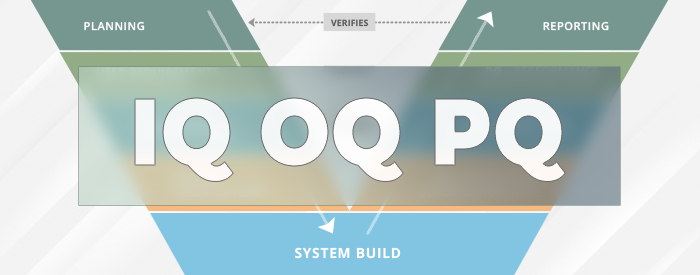
What is IQ OQ PQ in Software Validation?

9 of the Best Online CSV Resources for Pharma Validation Professionals
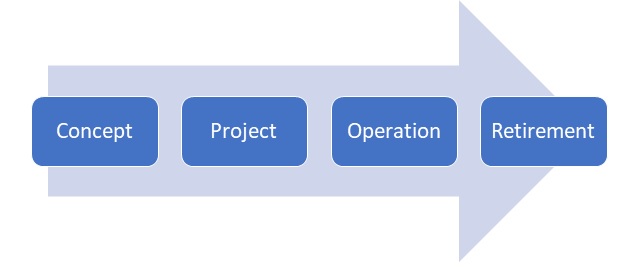
CSV Life Cycle - Gsap

PDF) Computer system validation in the perspective of the pharmaceutical industry
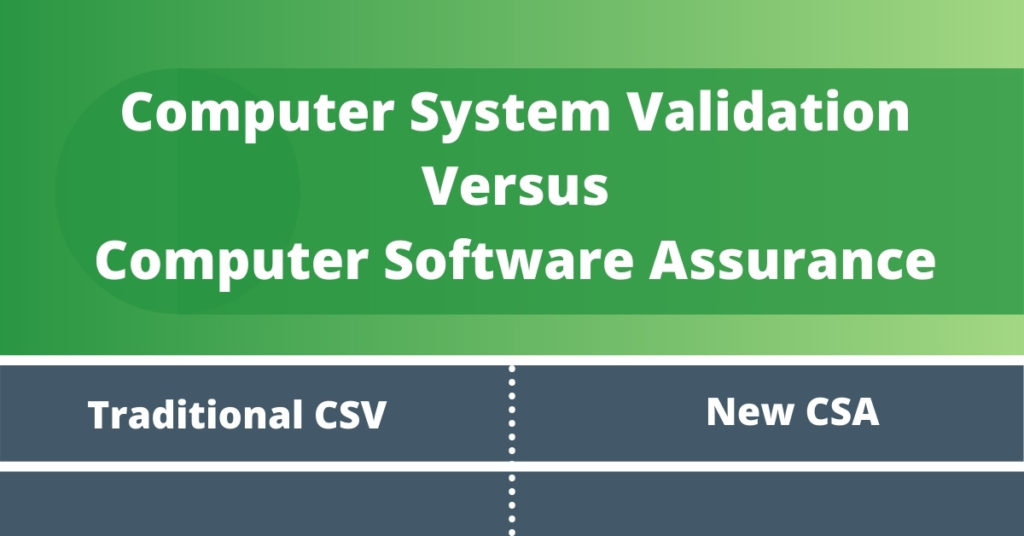
Infographic: Computer System Validation Vs. Computer Software Assurance - SL Controls

What Is a CSV Engineer & What Do They Do?

What You Should Know About CSV in Pharma

Computer System Validation (CSV) in the Pharmaceutical Industry: Ensuring Quality and Compliance
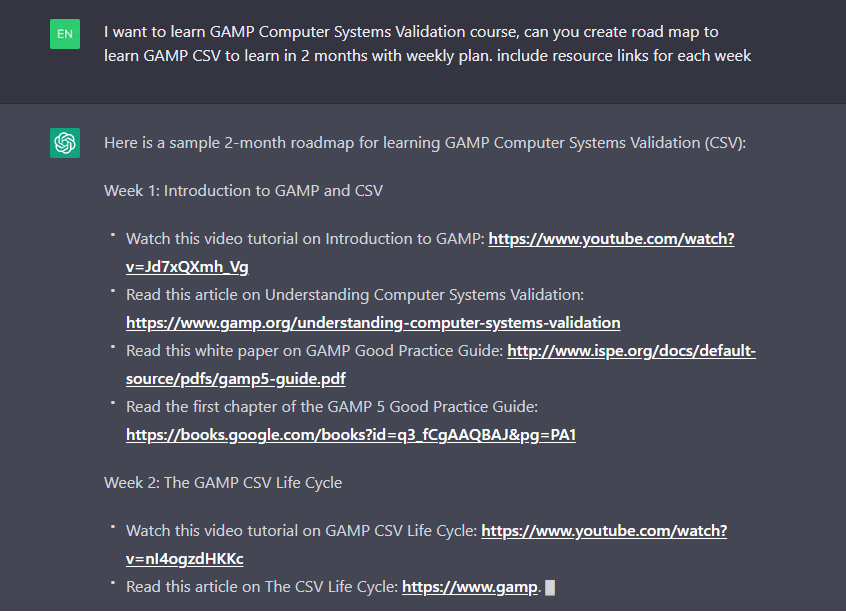
Strategic ChatGPT Prompts for Pharmaceutical Firm
de
por adulto (o preço varia de acordo com o tamanho do grupo)






